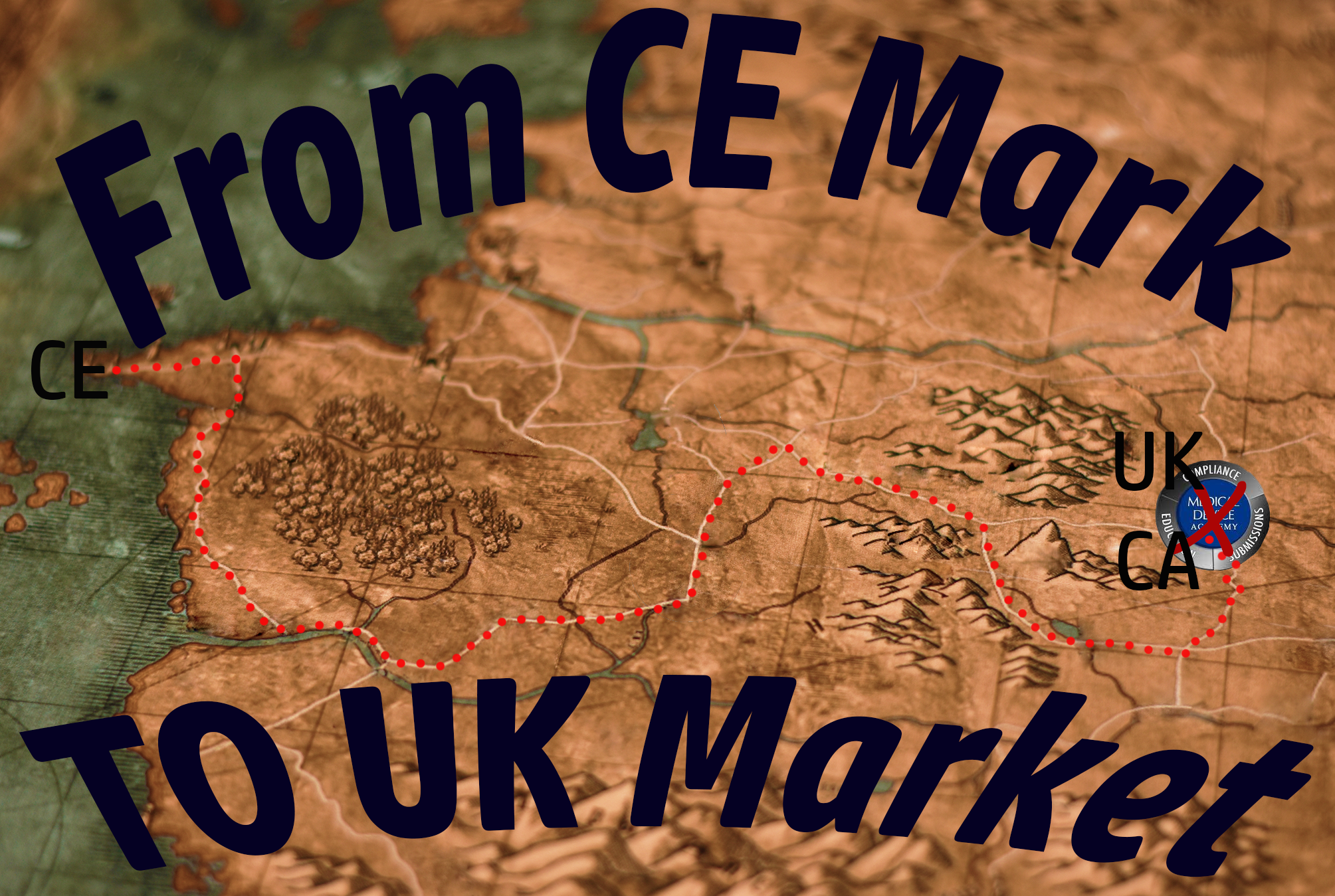
Brexit created a new medical device market, once CE Marked devices could be sold, new rules, and new marks now apply as the UKCA Mark is introduced!
On January 1st, 2021, the United Kingdom official exited the European Union as a participating member state. When this happened, it created a gap in the medical device community. Previously as a participating member state, the rules that applied to introducing a medical device to the market of the UK were the same rules for other EU countries, CE Marking, the introduction of the MDR and IVDR, the possible need for an EU Authorized Representative, etc. By leaving the EU, the UK has now introduced an entirely separate medical device market with new regulations and requirements for the approval needed to legally market your medical device. Devices in this market will now require a UKCA Mark.
From CE Mark to UKCA Mark
This training will outline the requirements for introducing your CE Marked device to the UK market including:
- Will CE Marks continue to be recognized?
- What about EU Notified Bodies?
- Defining what the UK Market is
- Device Registration
- UK Approved Bodies
- UK Responsible Person
- UKCA Mark
- What about Northern Ireland?
- Manufacturing within the UK versus Importing into the UK
- Labeling Requirements
- Post-Market Surveillance and Vigilance
The primary audience for this training is medical device manufacturers with or considering pursuing CE Marking for their medical devices, including IVDs, and Custom Made Devices that wish to expand into the UK market. We will also include potential changes and additions to your Quality Management System that may be required as well as identifying UK Guidance Documents that would assist you with entering the UK market and achieving a UKCA Mark.
What you will receive with this webinar:

- A video recording of the training
- Written Whitepaper on the topic
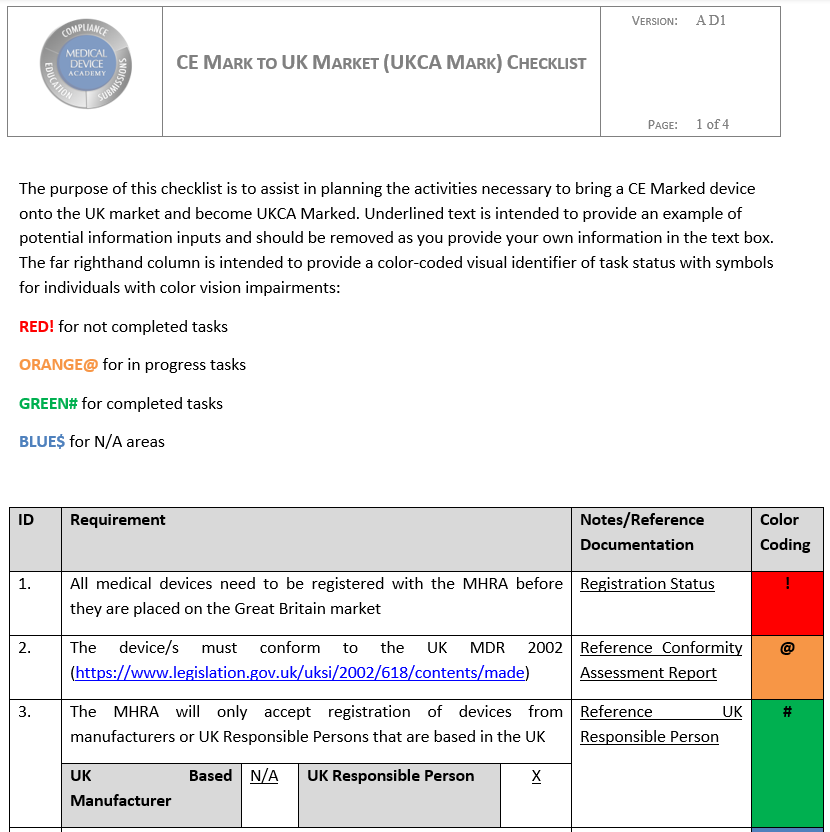
- CE Mark to UK Market Checklist to help plan your market expansion
- .PDF attestation certificate of participation for training records (A final test is provided for those that wish to receive a training certificate rather than a self-attested certificate of participation)
- URL and references to supporting guidance documents and regulations (where available, .PDFs will be provided but many guidance documents from www.gov.uk are provided in a browser-based web format. HTML versions can be converted to .PDF but the most up to date information will be found on the gov.uk website.)
Purchase the webinar for $129
We also provide an exam (i.e., a 10-question quiz) to verify training effectiveness. If you submit the completed exam to us by email in the native MS Word format, we will correct the exam and email you a training certificate with your corrected exam. Within your quality system, this record can be used to demonstrate the verification of training effectiveness and maintenance of qualifications and competencies. If you have more than one person that requires a training certificate, we charge $49/exam graded–invoiced upon completion of grading.
Please note: A link for downloading this webinar on the UKCA Mark will be delivered to the email address provided in the shopping cart transaction. After the transaction is verified, please check your email for receipt of the download link. To view, all available webinars click HERE.
Don’t forget to visit us on our Youtube Channel for regular videos regarding medical device regulatory affairs/submissions and quality management system content HERE.