Quality system auditing is outsourced to consultants providing auditing services to ensure auditor independence. Do you need a quote?
Who quotes auditing services?
The form below provides us with the basic information we need to prepare an auditing services quote for your company. There are instructions below the form that explain exactly what information we are looking for in each section of the form. The quotation process is not automated. A real person (i.e. Lindsey Walker) will get back to you with a quotation. She is our audit program manager. She creates the audit quote and assigns the auditors based on availability and your auditing needs. Her email is sales@medicaldeviceacademy.com. The quotation will be automatically emailed from Freshbooks once she is finished, and then she will follow up with a manual email–just in case your spam filters prevent delivery of the automated email generated by FreshBooks. If Lindsey is on vacation, or out sick, the proposal will be prepared by Rob Packard. His email is rob@13485cert.com.
General pricing of auditing services
If you are looking for the cheapest auditing services you can find, don’t even bother filling in the form. Our goal is to help you improve your quality system and provide valuable consulting advice to achieve improvements. We specialize in helping start-up companies achieve initial ISO 13485 certification, MDSAP certification, and CE Certification. We will assign an experienced lead auditor with an hourly consulting rate of $275/hour. Typically, we will charge $2,750 plus travel expenses for a one-day supplier audit because we expect to spend 30 minutes on audit preparation, eight hours on-site actively auditing, and 2+ hours generating an audit report. Most quotations are flat-fee quotations so you know exactly how much you will be charged. We also request a 50% deposit for audits.
Name, Company, Email & Phone
What is the audit type?
- Internal Audit – This is also called a “1st party audit,” and these are conducted to evaluate the effectiveness of your quality system. You are required to conduct an audit of the full quality system each year. If you select “Internal Audit,” we will assume that you want us to provide an audit quote for your complete quality system. If you only want a partial quality system audit of one or more process areas, then please select “Individual Process” and specify which process or processes in the text box labeled “Process Areas to Audit.”
- Supplier Audit – This is also called a “2nd party audit,” and these are conducted to evaluate the effectiveness of your supplier’s quality system. Other reasons for a supplier audit include verifying compliance with contractual requirements or identifying the root cause of a quality problem (i.e. nonconforming product). Please provide the details of what processes to audit in the text box labeled “Process Areas to Audit.” We generally recommend focusing supplier audits on the activities you are outsourcing (e.g. manufacturing) rather than general quality system requirements (e.g. management review).
- Individual Process Audits – This is also a “1st party audit,” however, we will focus on one or more processes that you identify in the text box labeled “Process Areas to Audit.” This type of audit is ideal when you do not have a qualified auditor that is independent to audit a process. Another scenario where this type of audit is valuable is when you recently made a significant change to a process and you want to verify that the employees are following the new process, or if you want to verify the effectiveness of corrective actions implemented for a specific process. For example, you want to verify the effectiveness of a CAPA related to an FDA 483 or Notified Body Nonconformity.
Process areas that need auditing
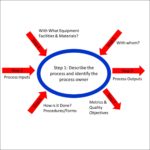 In this text box, we need you to identify the process areas you want us to audit. You can ask us to audit just one process or multiple processes. For example, if you are the Quality Manager and the only qualified lead auditor in your company, you might want us to audit your internal auditing, CAPA, management review, control of documents, and control of records. For a single process audit, we generally recommend remote audits via Zoom in order to eliminate the cost of travel. This is also a great way to test us before you engage our firm for a full-quality system internal audit. This is also known as the “audit scope,” and should not be confused with “audit criteria” discussed below. The scope can also include the location of the audit.
In this text box, we need you to identify the process areas you want us to audit. You can ask us to audit just one process or multiple processes. For example, if you are the Quality Manager and the only qualified lead auditor in your company, you might want us to audit your internal auditing, CAPA, management review, control of documents, and control of records. For a single process audit, we generally recommend remote audits via Zoom in order to eliminate the cost of travel. This is also a great way to test us before you engage our firm for a full-quality system internal audit. This is also known as the “audit scope,” and should not be confused with “audit criteria” discussed below. The scope can also include the location of the audit.Location (remote or on-site) for auditing services quote
 If you want us to conduct the audit remotely via Zoom, please enter “Remote” in the text box of the auditing services quote form. You can also specify another teleconferencing software of your choice. In general, we recommend that remote audits be split into 90-minute segments or less where one or two processes are covered during the 90-minute Zoom meeting. We explain this further in one of our blog articles: “Why remote audit duration should never exceed 90 minutes.” If you want us to conduct the audit on-site, please provide the address of the audit location and we will include the estimated travel costs in our proposal.
If you want us to conduct the audit remotely via Zoom, please enter “Remote” in the text box of the auditing services quote form. You can also specify another teleconferencing software of your choice. In general, we recommend that remote audits be split into 90-minute segments or less where one or two processes are covered during the 90-minute Zoom meeting. We explain this further in one of our blog articles: “Why remote audit duration should never exceed 90 minutes.” If you want us to conduct the audit on-site, please provide the address of the audit location and we will include the estimated travel costs in our proposal.Desired Date or Dates
 Please enter the date or dates that you want us to conduct your audit. You can also specify before a specific deadline (e.g. before June 30th). If you want us to conduct an audit of multiple processes remotely, it would help to know what dates and or times of day you would prefer. You can also enter a phone number and say “call me” next to the phone number. Then Lindsey or one of our assigned auditors will contact you to schedule a date and time for your audit.
Please enter the date or dates that you want us to conduct your audit. You can also specify before a specific deadline (e.g. before June 30th). If you want us to conduct an audit of multiple processes remotely, it would help to know what dates and or times of day you would prefer. You can also enter a phone number and say “call me” next to the phone number. Then Lindsey or one of our assigned auditors will contact you to schedule a date and time for your audit. What is the audit duration in hours?
 Please enter the desired duration of the auditing services you want to be quoted. We typically expect at least 30 minutes of audit preparation to review the audit preparation documents that you provide and to create an audit agenda. In addition, we expect to spend approximately two hours of report writing time for each eight-hour day of auditing. Therefore, a typically one-day supplier audit will require a duration of ten hours, while a three-day on-site internal audit will require a duration of 30 hours.
Please enter the desired duration of the auditing services you want to be quoted. We typically expect at least 30 minutes of audit preparation to review the audit preparation documents that you provide and to create an audit agenda. In addition, we expect to spend approximately two hours of report writing time for each eight-hour day of auditing. Therefore, a typically one-day supplier audit will require a duration of ten hours, while a three-day on-site internal audit will require a duration of 30 hours.Auditing criteria for auditing services quote
 It is important to specify the audit criteria for your auditing services quote, because otherwise, we might assign an auditor that does not have training on that criteria. Audit criteria are the standards, regulations, procedures, and contracts that may be used to evaluate your quality system or an individual process. Most of our audit team is qualified to audit against the following criteria:
It is important to specify the audit criteria for your auditing services quote, because otherwise, we might assign an auditor that does not have training on that criteria. Audit criteria are the standards, regulations, procedures, and contracts that may be used to evaluate your quality system or an individual process. Most of our audit team is qualified to audit against the following criteria:- 21 CFR 820, 803, 806, and 830 – the US FDA regulations including medical device reporting, corrections and removals, and unique device identifier regulations
- ISO 13485:2016/Amd 2021 – the international quality system standard for medical device manufacturers
- Regulation (EU) 2017/745 – the European Medical Device Regulations
- SOR 98/282 – the Canadian Medical Devices Regulation
- MDSAP AU P0002.008 – the Medical Device Single Audit Program audit approach guidance document
