 Why you should register for the IFU Validation Webinar
Why you should register for the IFU Validation Webinar
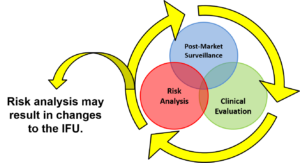
Most companies create an IFU for a new product by plagiarism. They merely copy a competitor’s IFU and change the name. If the IFU is created by a regulatory expert, the IFU will be nearly identical to the competitor IFU. However, if the IFU is created by a marketing person, the IFU will explain how your product is total different from the competitor product. Neither approach is effective. In the current regulatory environment you must use a risk-based approach, perform IFU validation and monitor post-market surveillance to determine if your IFU needs to be changed. In this webinar you will learn:
- How to develop your IFU using a risk-based approach
- Methods for IFU validation
- New Tools for gathering post-market surveillance and PMCF data
- How to revise and revalidate your IFU
What you will receive for $49
- a recording of the webinar you can replay anytime (mp4 format)
- the most recent version of my template for a Draft IFU (Word doc)
- the native slide deck for this webinar (PPT format)
There is a 28 slide presentation, and the presentation is 45 minutes in duration. Content is sent via AWeber emails to confirmed subscribers.
IFU Validation Webinar ($49)
When is the IFU Validation Webinar?
This presentation was recorded live on Friday, April 21, 2017, but you can purchase the native slide deck and the recording anytime.
Q&A
Please submit questions to me by email at rob@13485cert.com regarding the IFU Validation Webinar. If you have company-specific questions, please send me a request to set-up a private call to discuss your specific issues.
About Your Instructor
Rob Packard is a regulatory consultant with ~25 years experience in the medical device, pharmaceutical and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Rob was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certification. From 2009-2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Rob’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone 802.281.4381 or email. You can also follow him on Google+, LinkedIn or Twitter.