How do you demonstrate training competence?
Anyone can sign and date training records, but how do you demonstrate the effectiveness of training and competence?

What are the requirements for training?
The requirements for training are found in ISO 13485:2016, Clause 6.2 (i.e., Human Resources). Auditors and inspectors usually only ask for training records, but the requirement for records is the last item in the clause (i.e., “6.2(e) – maintain appropriate records of education, training, skills, and experience (see 4.2.5).”). The first four items in Clause 6.2 all include one word, but it’s not “records.” The most critical word in the requirements is “competence.”
What is the difference between training requirements, training records, training effectiveness, and training competence?
- Training requirements define the “appropriate education, training, skills, and experience.” Specifically, what degree(s) is required, if any? What training curriculum needs to be completed? What skills are needed to perform the work? And how many years of experience are needed?
- Training records are documented evidence that all training requirements have been met. Records may include a job application, resume, individual training records, group training records, or a training certificate. Records may also include the results of any quizzes to demonstrate training effectiveness and any evaluation of training competence.
- Training effectiveness is how well a person understands the information communicated during training. Using terms from human factors engineering (i.e., the PCA Process), the person must have the correct perception and cognition. Cognition can be evaluated by giving people quizzes or asking them questions. Written and verbal exams may also include pictures and/or video. Performing these types of tasks on a quiz or exam are “knowledge tasks.”
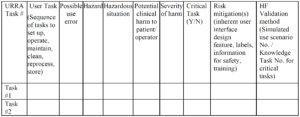
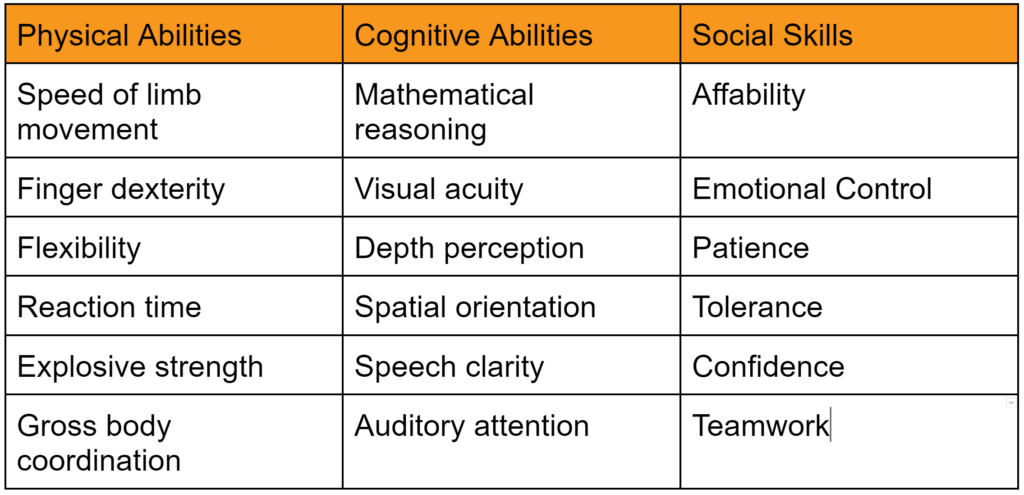
- Training competence is possessing the skill or ability to perform tasks. To demonstrate competence, someone that is already competent (i.e., a subject matter expert) must observe the trainee performing tasks. A person that once possessed the skill or ability can also judge competency, and a person that does not possess the skill or ability can be trained to evaluate skill and ability (e.g., an Olympic Judge or referee). Skills and abilities can be physical or cognitive, but there are also social skills. Examples of each are provided in the table below:
What keeps me awake at night? (a story from April 2, 2011)
I am in Canada, it’s almost midnight, and I can’t sleep. I’m here to teach a Canadian client about ISO 14971—the ISO Standard for Risk Management of medical devices. Most companies requesting this training are doing so for one of two reasons: 1) some design engineers have no risk management training, or 2) the engineers have previous training on risk management, but the training is out-of-date. This Canadian company falls into the second category, and engineers with previous training ask the most challenging questions. This group of engineers forced me to re-read the Standard several times and reflect on the nuances of almost every single phrase. While teaching this risk management course, I learned more about risk management than I ever knew.
The four levels of the Learning Pyramid
The image at the beginning of this blog is a learning model that explains my experiences training Canadian design engineers. I call this model the “Learning Pyramid.” At the base of the pyramid, there are “Newbies.”
This is the first of four levels. At the base, students read policies and procedures, hoping to understand.
The student is now asked to watch someone else demonstrate proper procedures in the second level of the pyramid. One of my former colleagues has a saying that explains the purpose of this process well, “A picture tells a thousand words, but a demonstration is like a thousand pictures.” Our children call this “sharing time,” but everyone over 50 remembers this as “show and tell.”
The student is now asked to perform their tasks in the third level of the pyramid. This is described as “doing,” but in my auditing courses, I refer to this process as “shadowing.” Trainees will first read the procedures for Internal Auditing (level 1). Next, trainees will shadow the trainer during an audit to demonstrate the proper technique (level 2). During subsequent audits, the trainees will audit, and the trainer will shadow the trainee (level 3). During this “doing” phase, the trainer must watch, listen, and wait for what I call the “teachable moment.” This is a moment when the trainee makes a mistake, and you can use this mistake as an opportunity to demonstrate a complex subject.
Finally, in the fourth level of the Learning Pyramid, we now allow the trainee to become a trainer. This is where I am at. I am an instructor, but I am still learning. I am learning what I don’t know.
Teaching forces you back to the bottom of the Learning Pyramid
After teaching, the next step in the learning process is to return to the first level. I re-read the Standards and procedures until I understood the nuances I was unaware of. Then, I search for examples in the real world that demonstrate the complex concepts I am learning. After searching for examples, I tested my knowledge by applying the newly acquired knowledge to an FDA 510(k) or De Novo submission for a medical device client. Finally, I prepared to teach again. This reiterative process reminds me of the game Chutes and Ladders, but one key difference is that we never really reach the level of “Guru.” We continue to improve but never reach our goal of perfection.
Where is training competence in the Learning Pyramid?
Most people feel that a person is competent in a position when they can consistently perform a task. However, the ISO 13485 standard uses the phrase “necessary competence” to suggest that competency for any given task might not be the same for all people. For example, in some cases, it may be sufficient to perform a task with written instructions in front of you, while an instructor might be expected to perform the task without written instructions. The speed at which a person performs a task might also differ. For example, a secretary might be required to touch type at a speed of 60+ wpm with a QWERTY keyboard, while a stenographer must be able to use a STENO keyboard and write at 225 wpm. The accuracy requirements may differ for those two positions as well. Therefore, your company may decide that the training competence requirement for a design engineer is that they can pass an exam on risk management. However, the training competency requirement for the design project lead or Engineering Manager might include teaching inexperienced design engineers to apply the basic risk management principles. Demonstrating the ability to teach inexperienced design engineers might be demonstrated by an auditor interviewing members of your design team.
How do you demonstrate training competence? Read More »