The author describes four tools (Five Why Analysis, Is/Is Not Analysis, Fishbone Diagram, and Pareto Analysis) and how each one can help conduct effective root cause analysis.
Quality problems are like weeds. If you don’t pull them out by the root, they grow right back.
Training on the 4 Tools
Most companies are doomed to repeat their mistakes because the root cause of their mistakes is not fixed. Why don’t companies fix their mistakes? Because the people responsible for the corrective actions (CAPA), were not adequately trained on root cause analysis. Adequate training on root cause analysis requires three things:
- Courage to admit that your process is broken
- Learning more than one tool for analyzing problems
- Practicing the use of root cause analysis tools
If your auditor identifies a nonconformity and you disagree with the finding, then you should not accept the finding and state your case. If an inspector rejects a part, and you believe the part is acceptable, then you should allow the part to be used “as is.” In both of these cases, however, you need to be very careful. Sometimes the problem is that “acceptable” is not as well-defined as we thought. I recommend pausing a moment and reflecting on what your auditors and inspectors are saying and doing. You may realize that you caused the problem.
Once you have accepted that there is a problem, you need to learn how to analyze the problem. There are five root cause analysis tools that I recommend:
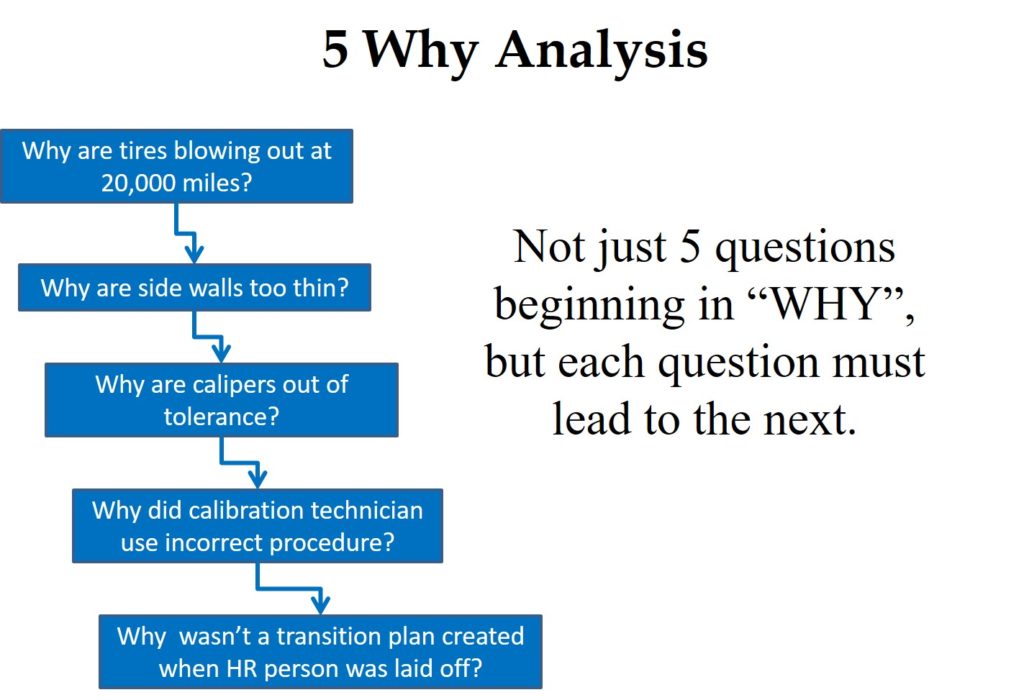
Root Cause Analysis Tool # 1 – 5 Why Analysis
A “Five Why Analysis” is not just five questions that begin with the word “why.” Taiichi Ohno is credited with institutionalizing the “Five Why Analysis” at Toyota as a tool to drill down to the root cause of a problem by asking why five times. I have read about this, used this tool, and taught this concept to students, but I learned of a critical instruction that I was missing when I read Toyota Under Fire.
In that book, Jeff Liker makes the following statement, “Toyota Business Practices dictates using the ‘Five Whys’ to get to the root cause of a problem, not the ‘Five Whos’ to find a fire the guilty party.” At the end of the book, there are lessons learned from Toyota’s experience. Lesson 2 says, “There is no value to the Five Whys if you stop when you find a problem that is outside of your control.” If your company is going to use this tool, it is important that the responsible person is the one performing the five why analysis, and asks why they didn’t take into account forces that are out of their control.
Root Cause Analysis Tool # 2 – Is/Is Not Analysis
The next tool was presented to me at an AAMI course that I attended on CAPA. One of the instructors was from Pathwise, and he explained the “Pathwise Process” to us for problem-solving. A few years later, I learned that this tool is called the “Is/Is Not Analysis.” This tool is intended to be used when you are having trouble identifying the source of a problem. This method involves asking where the problem is occurring as a potential clue to the reason for the problem. For example, if the problem only occurs on one machine, you can rule out a lot of possible factors and focus on the few that are machine-specific.
The reverse approach is also used to help identify the cause. You can ask where the problem is not occurring. This approach may also lead you to possible solutions to your problem. For example, if the problem never occurs on the first or second shift, you should focus on the processes and the people that work on the third shift to locate the cause. The “Is/Is Not Analysis” is seldom used alone, but it may be the first step toward locating the cause of a quality problem.
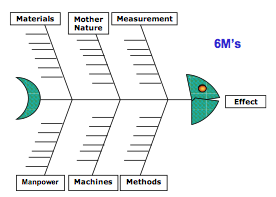
Root Cause Analysis Tool # 3 – Fishbone Diagram
This name comes from the shape of the diagram. Other names for this diagram are the “Cause and Effect” or “Ishikawa” diagram. If a problem is occurring in low frequency and has always existed, this might not be your first tool. However, I typically start with this tool when I am doing an investigation of nonconforming product—especially when rejects suddenly appear.
If you are baffled about the cause of a problem, brainstorming the possible causes in a group sometimes works. However, I like to organize and categorize the ideas from a brainstorming session into the “6Ms” of the Fishbone Diagram.
Root Cause Analysis Tool # 4 – Pareto Analysis
The fourth root cause analysis tool is the Pareto Analysis named after Antoine Pareto. This tool is also a philosophy that was the subject of a book called The 80/20 Principle: The Secret to Achieving More with Less. The Pareto Analysis is used to organize a large number of nonconformities and prioritize the quality problems based upon the frequency of occurrence. The Pareto Chart presents each challenge in descending order from the highest rate to the lowest frequency. After you perform your Pareto Analysis, you should open a CAPA for the #1 problem, and then open a CAPA for the #2 problem. If you get to #3, consider yourself lucky to have the time and resources for it. We have an example of a Pareto Chart in our article on FDA 483 inspection observations from 2013.
Additional Training Resources
If you are interested in learning more about root cause analysis and practicing these techniques, please register for the Medical Device Academy’s Risk-Based CAPA training.

Pingback: packaging complaint investigation
Pingback: 15 Tips for Creating an Effective CAPA Form -
Pingback: 8 Steps to Writing a CAPA Procedure Compliant with ISO 13485:2016 -
Hi Rob,
I am not able to visualize myself on IS/Is not analysis on my job for problem such as visual defect.Is it possible to provide examples of how to do IS/Is not analysis in your blog.
For example, at what stage of your process flow do visual defects appear? You might also look to see what shifts never have visual defects or which operators never have visual defects. If you can figure out where the problem is not occurring, that will help you narrow down the root cause.
I think there is value in the other models you listed but I can’t stand the Pareto principle (particularly the name which is trying to give this pseudoscience credibility when Pareto himself had nothing to do with it). Even where it is not grossly misapplied it is a ridiculous rule of thumb that is most likely only ever coincidentally “accurate”.
I agree that it is probably only a coincidence that most problems are caused by 20% of issues. It is just as likely that the number of issues that cause 80% of the problems is 15% or 25%. However, the basic process of sorting your quality problems from the most common to the least common problem is extremely helpful in focusing your CAPA efforts. In general, I focus on the biggest problem first, then the second biggest problem. If I get to #3, I’m damn lucky. I also have seen many cases where the biggest problem is categorized as “no trouble found.” That tells me the problem was never even solved.
RCA helps to recognize factors that contribute to the problem and to avoid the temptation of resolving the problem as fast as possible by pointing to the most obvious ‘Culprit’. You really want to find the real story, the origin of the problem as opposed to just reviewing and resolving symptoms.
Check this article: https://imdcr.com/how-to-perform-rca-4-easy-steps/#comment-554
I certainly agree with your comments about root cause analysis, but the article you provided a link to is very short and doesn’t really provide any tools for root cause analysis. Also, I haven’t seen too many cases where I would say that a well-down root cause analysis was “easy.”
Pingback: How to Utilize CAPA Training To Avoid FDA 483 Citations Medical Device Academy
Pingback: CAPA Form - 15 tips to avoid CAPA failure Medical Device Academy
Pingback: CAPA Training Remediation Case Study: Hundreds of Open CAPAs - Medical Device Academy Medical Device Academy
Pingback: Effective Management Solutions for 10 CAPA Program Blunders - Medical Device Academy Medical Device Academy
Pingback: FDA 483 Inspection Observations Pareto Chart for FY 2015 Data - Medical Device Academy Medical Device Academy
Pingback: CAPA procedure, How do you improve quality and prevent nonconformity? Medical Device Academy
Pingback: CAPA Case Study - Medical Device Academy Medical Device Academy