Medical Device Academy’s ISO 14971:2019 risk management training webinar is being expanded from a single webinar to a two-part webinar.
What’s new in this risk management training webinar?
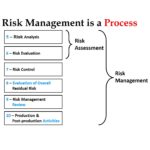
Our previous version of the ISO 14971 risk management training webinar was recorded on October 19, 2018. Although that webinar is 100% compliant with the 2019 version of the ISO 14971 standard, everyone considering the purchases of that webinar asks me to confirm this. The webinar was possible because we were using the draft version of the standard for the webinar. Over time we observe clients struggling with the implementation of the risk management process. It is a complex process, and covering the topic in a single webinar is not really feasible. Therefore, we are updating the webinar again as a 2-part series. Part 1 will cover Clause 1 through Clause 7.1, while Part 2 will cover Clause 7.2 through Clause 10. We have selected Clause 7.2 to begin Part 2 of this webinar series because it marks the beginning of the verification of the risk controls your company has implemented (i.e. – Post “Design Freeze”).
When is the risk management training webinar?
Part 1 of this webinar will be presented live on Tuesday, March 29 @ 9-10:30 am EDT. Part 2 of this webinar series will be presented live on Tuesday, April 5 at 9-10:30 am EDT. Purchase of this webinar series will grant the customer access to both live webinars. They will also receive the native slide decks and recording for the two webinars. We are also updating our quiz for training effectiveness from a 10-question quiz to a 20-question quiz that is more comprehensive. Any person that completes the 20-question quiz will receive a training certificate, a corrected quiz, and the answer key to our quiz. All turnkey quality system customers will receive access to this updated webinar series, and existing turnkey customers will be invited to participate in the live webinars. Customers that already purchased the recording from October 19, 2018, will receive invitations to participate in the live webinars too.
Register for the Risk Management Training Webinar for $129.00 (USD)
A risk management procedure compliant with ISO 14971 is the best practice for meeting the requirement in ISO 13485:2016, Clause 7.1. If you are unfamiliar with the ISO 13485 standard, please visit our page on “What is ISO 13485?“
The topic of the month for April is Risk Management. The risk management procedure, SYS-010, will be available at a 50% discount if you use the “ISO 14971” discount code (March 21, 2022 – April 20, 2022).
VIEW OUR RISK MANAGEMENT PROCEDURE
CLICK HERE OR IMAGE BELOW:
About Your Instructor
Robert Packard is a regulatory consultant with ~25 years of experience in the medical device, pharmaceutical, and biotechnology industries. He is a graduate of UConn in Chemical Engineering. Robert was a senior manager at several medical device companies—including President/CEO of a laparoscopic imaging company. His Quality Management System expertise covers all aspects of developing, training, implementing, and maintaining ISO 13485 and ISO 14971 certifications. From 2009 to 2012, he was a lead auditor and instructor for one of the largest Notified Bodies. Robert’s specialty is regulatory submissions for high-risk medical devices, such as implants and drug/device combination products for CE marking applications, Canadian medical device applications, and 510(k) submissions. The most favorite part of his job is training others. He can be reached via phone at 802.258.1881 or by email. You can also follow him on Google+, LinkedIn, or Twitter.