The 510k course includes 25 new eSTAR webinars, 36+ historical webinars, electronic templates for your next 510k submission, and the eBook “How to Prepare a 510(k) in 100 days.”
The primary advantage of purchasing this 510k Course is that you and your team will receive invitations to new webinars whenever the FDA updates the requirements for a 510k or De Novo submission–at no additional cost. We will also update our existing webinars and templates as we receive feedback from FDA reviewers during the technical screening process, substantive reviews, and interactive reviews. When you purchase our 510k course, you will receive an automated email asking you to confirm your desire to join an email subscription. We deliver all of the updated content through AWeber email subscriptions. If you do not subscribe, you will not receive the updated content. The first email you will receive is to thank you for purchasing and subscribing to email updates. The email includes a link to two Dropbox folders (contents as of 1-25-2024):
You will need sufficient Dropbox storage space to access the content, but the content can be downloaded and saved locally.
Buy the 510k course for $1,495
Note: If you purchased our new FDA PreSTAR tutorial ($299), you will receive a credit for that prior purchase. Just email Becca Taylor, asking her to email you an invoice for the lower amount, or she can issue a partial refund.
What are the contents of the “510k Template” folder in the 510k Course?
Obsolete 510k templates not intended for use with the FDA eSTAR templates have been archived permanently, and the volume/document structure is no longer used for 510k and De Novo submissions. The screen capture below shows the updated templates folder structure. There are 20 primary templates in the folder–including the two eSTAR templates and three financial disclosure forms provided by the FDA. Most of the content provided in templates is now incorporated into the eSTAR. If you are looking for software or cybersecurity documentation templates, those are sold separately:
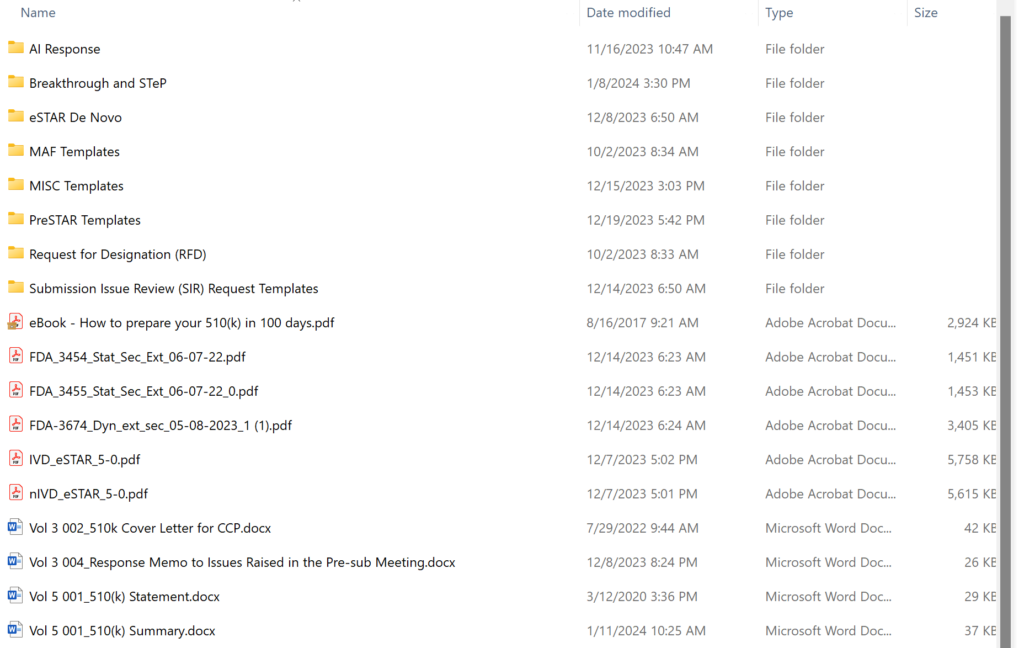
What are the contents of the “510k and De Novo Webinars” folder for the 510k course?
The 510k and De Novo Webinars folder includes all our current and past webinars on 510k submissions. The list below identifies which webinars can be purchased separately and which are only available as part of the 510k Course. The first 26 webinars are listed in the order of the FDA eSTAR from beginning to end.
- FDA eSTAR General Overview – Only available as part of 510k Course
- FDA PreSTAR Tutorial (4-part webinar series) – $299
- Consensus Standards Webinar – $79 (March 14, 2024)
- Device Description Webinar – $29 (November 17, 2022)
- Indications for Use Webinar – $64.50 (December 1, 2022)
- Predicate Selection Webinar – $64.50 (December 8, 2022)
- Special 510(k) Webinar – $79 (March 28, 2024)
- Labeling & UDI 510k Requirements Webinar – $49
- Reprocessing Webinar – Only available as part of 510k Course
- Sterilization Validation Webinar – $79 (April 25, 2024)
- Shelf-Life Webinar – $79 (March 21, 2024)
- Biocompatibility Webinar – $79 (February 29, 2024)
- 510k Software Documentation Webinar – Free Download (March 7, 2024)
- Cybersecurity Testing Webinar – Only available as part of 510k Course
- Interoperability Testing Webinar – Only available as part of 510k Course
- RF Wireless Technology Webinar – Only available as part of 510k Course
- EMC Testing Webinar – Only available as part of 510k Course
- Electrical Safety Testing Webinar – $79 (January 18, 2024)
- Human Factors Webinar – Only available as part of 510k Course
- 510(k) Benchtop Performance Testing Webinar – $79 (January 25, 2024)
- 510(k) Summary – $79 (January 11, 2024)
- Additional Information Request Webinar – Only available as part of 510k Course
- Animal Performance Testing Webinar – $79 (February 1, 2024)
- Human Clinical Study Webinar – $79 (February 8, 2024)
- Dual 510(k) and CLIA Waiver Webinar – $79 (February 15, 2024)
- Test Plan Webinar – $79 (February 22, 2024)
- PreSTAR v1.0 513(g) Template – $79 (April 5, 2024)
Other 510k Course Webinars
- 513g Submission Webinar Bundle – $79 (April 5, 2024)
- 510k Submissions: Substantial Equivalence – $129
- FDA eCopy Print & Ship Webinar – Free if you submit a question
- 510k FAQs Webinar – Free if you submit a question
- How to prepare your 510k in 100 days – only available through our 510k Course
- De Novo Application – $49 (September 11, 2022)
- Small Business Qualification Fee for 510(k) Submission – $19
- Redacted FOIA 510k Webinar – $79
- How to Complete a 510k Cover Letter Webinar – $29
- 510k Project Management Best Practices – Free
- 510k Project Management – Updated for 2021 – Free
- How To Combine a 510k Submission with CE Marking Technical Files – $64.50
- In Vitro Diagnostics (IVD) 510k Webinar – $29
- FDA eCopy Print & Ship Webinar – Free if you submit a question
Two new eSTAR templates were released on January 8, 2024, and the v4.3 eSTAR templates become invalid on February 4, 2024:
- nIVD eSTAR v5.2 (2024-03-29): The inclusion of more than 10 standards will no longer cause an Import to run very slowly. An attachment counter was added in the Verification section. A workaround was added to allow the proper display of an eSTAR in Adobe Acrobat Pro when it was prepared with PDF-XChange Editor and formatted text was copied to a textbox. A new international Help Text model was implemented in the Cover Letter and Labeling section to obtain feedback. The wireless function security question was removed from the Wireless Technology section, since it was redundant with content in the Cybersecurity section. AI/ML help text was added to the Software Description attachment question.
- Updated 5.1 (2024-01-08): Hydrogen Peroxide updated as an Established Category A sterilization method, consistent with latest Sterility Guidance.
- Update 5.0 (2023-12-06): PMA content enabled for FDA. Initial international pilot updates (not publicly available yet). EMC Labeling consolidated into a single question, due to typical single citation provided. Guide for Cybersecurity guidance document content added. Pyrogenicity testing options, help text, and EtO residuals question updated in Sterility section. Popup message added if Adobe Acrobat Reader is used. ISO 18562 information added to Biocompatibility section. Cross-section change reminders added to help texts in Device Description section. Clinical Testing section will now display when using PDF-XChange Editor.
- IVD eSTAR v5.2 (2024-03-29): The inclusion of more than 10 standards will no longer cause an Import to run very slowly. An attachment counter was added in the Verification section. A workaround was added to allow the proper display of an eSTAR in Adobe Acrobat Pro when it was prepared with PDF-XChange Editor and formatted text was copied to a textbox. A new international Help Text model was implemented in the Cover Letter and Labeling sections to obtain feedback. The wireless function security question was removed from the Wireless Technology section, since it was redundant with content in the Cybersecurity section. AI/ML help text was added to the Software Description attachment question.
- Update 5.1 (2024-01-08): Hydrogen Peroxide updated as an Established Category A sterilization method, consistent with updated Sterility Guidance.
- Update 5.0 (2023-12-06): PMA content enabled for FDA. Initial International pilot updated (not publicly available yet). EMC Labeling consolidated into a single question, due to typical single citation provided. Guide for Cybersecurity guidance document content added. Pyrogenicity testing options, help text, and EtO residuals question updated in Sterility section. popup message added if Adobe Acrobat Reader is used. ISO 18562 information added to Biocompatibility section. cross-section change reminders added to Help Texts in Device Description section.
- PreSTAR v1.0 (2024-03-29): 513(g) submission content was enabled. The inclusion of more than 10 standards will no longer cause an Import to run very slowly. An attachment counter was added in the Verification section. A workaround was added to allow the proper display of an eSTAR in Adobe Acrobat Pro when it was prepared with PDF-XChange Editor and formatted text was copied to a textbox. The FAQ was updated. The list of standards, list of regulations, and list of product codes were updated. Other minor bugs were fixed and minor changes were implemented.
- Update 0.3 (2023-12-06) from version 0.2: Standard recognition number can now be used to autopopulate standard information. Updated Software guidance document link. Updated list of medical device product codes. Updated FAQ. Fixed minor bugs.
- Update 0.2 (2023-08-14) from version 0.1: Addition of PCCP submission topic category. Minor text changes.
- Updated 0.1 (2023-06-06): Initial beta release.
Without knowing what design stage you are in, we can’t tell you where to start watching our 510k course webinars. However, the following YouTube video might help you figure out where you are in the design process. The video has timestamps that allow you to advance and rewatch any video section. Many start-up companies try to begin the 510k process without a design plan. They intend to create the design plan and design history file (DHF) retrospectively after they submit their 510k submission. However, this is a mistake. You should always start your 510k project with a design plan. The primary reason is that it will help you create a realistic timeline for the overall project. The second most important reason for starting with design planning is that it will force you to schedule the testing required for verification and validation (V&V) testing and any pre-requisites that must be completed before you begin V&V testing. We have another form allowing you to download our design plan template and 510k table of contents. These two documents are the primary planning tools we recommend you use to plan your 510k project.
To give you an idea of what we have included in our 510k course, below is a YouTube video recorded with Joe Hage in October 2018 explaining how to prepare a 510k submission.


Dear Rob,
I would like to ask for a quotation for the 510(k) courses and a list of the content included.
We recently start working with a US 510(k) project, and I think it is good to get my knowledge on 510(k) up to date. I have attended a few of your free webinars and a RAPS SaMD training, very helpful to my professional development as a RA specialist.
I also have the question of update plan from your side, as FDA published quite a few guidance recently. If I purchase the course now, will I get free access to the update webinars and updated templates?
Thanks a lot.
May
Dear May,
Thank you for your inquiry. If anyone has trouble with purchases on our website, needs a quote, or a receipt is needed; you can request a quote, invoice, or receipt directly from Becca Taylor (becca@medicaldeviceacademy.com) or Lindsey Walker (sales@medicaldeviceacademy.com). I will personally send you an invoice for the 510(k) course in a few moments, and you are welcome to contact me directly to ask any technical questions.
Regarding updates – I already conducted a webinar this week about the predicate selection draft guidance. Another webinar is planned for next week on biocompatibility, but I have not updated the website yet. If you purchase the course now, you get invites to updated webinars and access to all of the recording in the future. Any new templates we create you will also have access to, but the number of templates is decreasing with the implementation of the eSTAR.
Rob